Controlled Substance List
The Controlled Substances Act (CSA) is the statute establishing federal U.S. Drug policy under. Subchapter I defines Schedules I-V, lists chemicals used in the manufacture of controlled substances, and differentiates lawful and unlawful. May 18, 2018 - A substance does not need to be listed as a controlled substance by the DEA to be treated as a Schedule I substance for criminal prosecution.
Marijuana
List of Controlled Substances
Lists of Scheduling Actions, Controlled Substances, Regulated Chemicals (PDF) (May 2019)
This document is a general reference and not a comprehensive list. This list describes the basic or parent chemical and does not describe the salts, isomers and salts of isomers, esters, ethers and derivatives which may also be controlled substances.
| Scheduling Actions | Controlled Substances | List I and II Regulated Chemicals |
|---|---|---|
| Alphabetical Order | Alphabetical Order | Alphabetical Order |
| Chronological Order | DEA Drug Code Number | DEA Number |
| CSA Schedule | List Number | |
| Illicit Uses and Threshold Quantities |
Exempted Lists
Exempt Anabolic Steroid Products
Exempt Anabolic Steroid Products Procedures
Exempt Anabolic Steroid Products List (PDF) (February 6, 2015)
Exempt Chemical Preparations
Exempt Chemical Preparations List (PDF) (November 7, 2017) For Application Dates Through December 31, 2016
Exempted Prescription Products
Exempted Prescription Products Application
Exempted Prescription Products List (PDF) (February 28, 2019)
Lists of Controlled Substances Disclaimer
Section 812 of the Controlled Substances Act (21 U.S.C. §801 et seq.) (CSA) lists substances which were controlled in 1970 when the CSA was enacted. Since then many substances have been added, removed, or transferred from one schedule to another. The current list of controlled substances can be found in section 1308 of the most recent issue of Title 21 Code of Federal Regulations (CFR) Part 1300 to end (21 CFR §1308) and the final rules which were published in the Federal Register subsequent to the issuance of the CFR.
These lists describe the basic or parent chemical and do not describe the salts, isomers, salts of isomers, esters, ethers, and derivatives which may be controlled substances. These are not comprehensive lists so please note that a substance need not be listed as a controlled substance to be treated as a scheduled substance for criminal prosecution. The 'Other Names' column, provides some examples of alternate names for certain compounds, and in some instances provides examples of 'positional isomers'. If outside parties want to ensure that a compound is not considered a scheduled substance or listed chemical, they should write the DEA, Drug and Chemical Evaluation Section (DRE), Diversion Control Division, 8701 Morrissette Drive, Springfield, Virginia 22152, for an official determination.
A substance (not included on these lists) may also be regulated as a controlled substance analogue. A controlled substance analogue is a substance which is intended for human consumption, is structurally substantially similar to a schedule I or schedule II substance, is pharmacologically substantially similar to a schedule I or schedule II substance, or is represented as being similar to a schedule I or schedule II substance and is not an approved medication in the United States. See 21 U.S.C. §802(32)(A) for the definition of a controlled substance analogue and 21 U.S.C. §813 for the schedule.
Defined Abbreviations
| Defined Abbreviation | Controlled Substance Analogue |
|---|---|
| 2C-B | 4-Bromo-2,5-dimethoxyphenethylamine |
| 2C-T-7 | 2,5-Dimethoxy-4(n)-propylthiophenethylamine |
| BZP | N-Benzylpiperazine |
| DMT | Dimethyltryptamine |
| DOM | 4-Methyl-2,5-dimethoxyamphetamine |
| GBL | Gamma butyrolactone |
| GHB | Gamma hydroxybutyric acid, gamma hydroxybutyrate, 4-hydroxybutanoic acid, sodium oxybate |
| LAAM | Levo-alphacetylmethadol |
| LSD | Lysergic acid diethylamide, lysergide |
| MDA | 3,4-Methylenedioxyamphetamine |
| MDE | 3,4-Methylenedioxy-N-ethylamphetamine |
| MDMA | 3,4-Methylenedioxymethamphetamine |
| MPPP | 1-Methyl-4-phenyl-4-propionoxypiperidine |
| P2P | Phenyl-2-propanone, phenylacetone |
| PCC | 1-Piperidinocyclohexanecarbonitrile |
| PCE | N-Ethyl-1-phenylcyclohexylamine |
| PCH | 1-Phenylcyclohexylamine |
| PCP | 1-(1-Phenylcyclohexyl)piperidine, phencyclidine |
| PEPAP | 1-(2-Phenylethyl)-4-phenyl-4-acetoxypiperidine |
| PHP | 1-(1-Phenylcyclohexyl)pyrrolidine |
| SPA | (-)-1-Dimethylamino-1,2-diphenylethane |
| TCP | 1-[1-(2-Thienyl)cyclohexyl]piperidine |
| TCPy | 1-[1-(2-Thienyl)cyclohexyl]pyrrolidine |
| THC | Tetrahydrocannabinols |
| THG | Tetrahydrogestrinone |
Definition of Controlled Substance Schedules
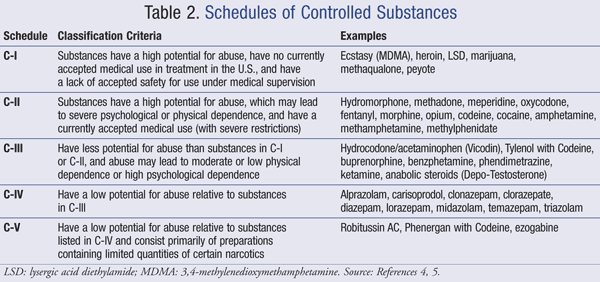
Drugs and other substances that are considered controlled substances under the Controlled Substances Act (CSA) are divided into five schedules. An updated and complete list of the schedules is published annually in Title 21 Code of Federal Regulations (C.F.R.) §§ 1308.11 through 1308.15. Substances are placed in their respective schedules based on whether they have a currently accepted medical use in treatment in the United States, their relative abuse potential, and likelihood of causing dependence when abused. Some examples of the drugs in each schedule are listed below.
Schedule I Controlled Substances
Substances in this schedule have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse.
Some examples of substances listed in Schedule I are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), peyote, methaqualone, and 3,4-methylenedioxymethamphetamine ('Ecstasy').
Schedule II/IIN Controlled Substances (2/2N)
Substances in this schedule have a high potential for abuse which may lead to severe psychological or physical dependence.
Examples of Schedule II narcotics include: hydromorphone (Dilaudid®), methadone (Dolophine®), meperidine (Demerol®), oxycodone (OxyContin®, Percocet®), and fentanyl (Sublimaze®, Duragesic®). Other Schedule II narcotics include: morphine, opium, codeine, and hydrocodone.
Examples of Schedule IIN stimulants include: amphetamine (Dexedrine®, Adderall®), methamphetamine (Desoxyn®), and methylphenidate (Ritalin®).
Other Schedule II substances include: amobarbital, glutethimide, and pentobarbital.
Schedule III/IIIN Controlled Substances (3/3N)
Substances in this schedule have a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low physical dependence or high psychological dependence.
Examples of Schedule III narcotics include: products containing not more than 90 milligrams of codeine per dosage unit (Tylenol with Codeine®), and buprenorphine (Suboxone®).
Examples of Schedule IIIN non-narcotics include: benzphetamine (Didrex®), phendimetrazine, ketamine, and anabolic steroids such as Depo®-Testosterone.
Schedule IV Controlled Substances
Substances in this schedule have a low potential for abuse relative to substances in Schedule III.
Examples of Schedule IV substances include: alprazolam (Xanax®), carisoprodol (Soma®), clonazepam (Klonopin®), clorazepate (Tranxene®), diazepam (Valium®), lorazepam (Ativan®), midazolam (Versed®), temazepam (Restoril®), and triazolam (Halcion®).
Schedule V Controlled Substances
Substances in this schedule have a low potential for abuse relative to substances listed in Schedule IV and consist primarily of preparations containing limited quantities of certain narcotics.
Examples of Schedule V substances include: cough preparations containing not more than 200 milligrams of codeine per 100 milliliters or per 100 grams (Robitussin AC®, Phenergan with Codeine®), and ezogabine.
The Drug Enforcement Administration (DEA) has announced its decision: It will keep marijuana in the same legal, regulatory category as heroin — schedule 1.
To many people, this is outrageous. Obviously, marijuana is nowhere as dangerous as heroin. And it's not more dangerous than schedule 2 drugs like cocaine and meth. So why the hell is pot schedule 1?
But the classification doesn't mean that the federal government thinks of marijuana and heroin as equally dangerous drugs. The schedule reflects a more complicated system — one that accounts for a drug's medical value as much as a drug's potential for abuse.
A harsh schedule also does not mean a drug is totally illegal. Criminal laws, while guided by the scheduling system, often take other factors into account. For pot, they do — leaving it as one of the less-punished illicit drugs at the federal level, even though it's schedule 1. And opioid painkillers, as one example, are schedule 2 but legal for medical purposes.
Still, a drug's schedule is an important policy guide. A stricter schedule lets the DEA more stringently limit access to a drug and its supply, which can make a drug more difficult to research — as has happened for marijuana, limiting researchers' ability to study the drug for its medical value. (As a result, advocacy and medical groups have long contended that pot's schedule is out of step with the available scientific evidence.)
So what is the scheduling system, how does it work, and what would it take to reschedule a drug? Here's what you need to know.
How does the US classify illicit drugs like marijuana?
Under the Controlled Substances Act, the federal government — which has largely relegated the regulation of drugs to the Drug Enforcement Administration (DEA) — puts each drug into a classification, known as a schedule, based on its medical value and potential for abuse.
To initiate a schedule, the DEA first asks if a drug can be abused. If the answer is yes, then it's put on a schedule. If no, the drug is left out. After that, the drug's medical value and relative potential for abuse are evaluated to decide where on the scale it lands.
The two big issues, then, are a drug's potential for abuse and its medical value. Congress did not clearly define abuse under the Controlled Substances Act. But for federal agencies responsible for classifying drugs, abuse is when individuals take a substance recreationally and develop personal health hazards or pose other risks to society as a whole. To find medical value, a drug must have large-scale clinical trials to back it up — similar to what the Food and Drug Administration (FDA) would expect from any other drug entering the market.
Schedule 1 drugs have no medical value and high potential for abuse, while schedule 2 through 5 substances all have some medical value but differ in ranking depending on their potential for abuse (from high to low).
Some examples of the drugs that are on each schedule:
- Schedule 1: marijuana, heroin, LSD, ecstasy, and magic mushrooms
- Schedule 2: cocaine, meth, oxycodone, Adderall, Ritalin, and Vicodin
- Schedule 3: Tylenol with codeine, ketamine, anabolic steroids, and testosterone
- Schedule 4: Xanax, Soma, Darvocet, Valium, and Ambien
- Schedule 5: Robitussin AC, Lomotil, Motofen, Lyrica, and Parepectolin
In general, schedule 1 and 2 drugs have the most regulatory restrictions on research, supply, and access, and schedule 5 drugs have the least.
Does the federal government really consider marijuana more dangerous than cocaine?
To many people, one of the more bewildering aspects of the scheduling system is that marijuana is schedule 1 — the same category as heroin — while cocaine and meth are schedule 2.
But that doesn't necessarily mean the federal government views marijuana and heroin as equally dangerous drugs, or that it considers marijuana to be more dangerous than meth or cocaine. Schedule 1 and 2 drugs are both described as having 'a high potential for abuse' — a vague description that doesn't rank drugs in the two categories as equal or different.
The big distinction between schedule 1 and 2 substances, instead, is whether the federal government thinks a drug has medical value. The DEA says schedule 2 substances have some medical value and schedule 1 substances do not, so the latter receive more regulatory scrutiny even though they may not be more dangerous.
It may be helpful to think of the scheduling system as made up of two distinct groups: nonmedical and medical. The nonmedical group comprises the schedule 1 drugs, which are considered to have no medical value and high potential for abuse. The medical group comprises the schedule 2 to 5 drugs, which have some medical value and are numerically ranked based on abuse potential.
There are some cultural considerations to the scheduling system, as well. The war on drugs was initiated at a time when much of the nation was in hysterics about what drugs like marijuana and LSD would do to the moral fabric of the country. Marijuana was seen as dangerous not necessarily because of its direct health effects, but because of the perception — partially rooted in racial prejudices — that pot makes people immoral, lazy, and even violent. This perception persists among many supporters of the war on drugs to this day, and it's still reflected in America's drug scheduling.
Beyond the scheduling system, the federal government imposes criminal trafficking penalties for drugs that don't always align with their scheduling. For instance, marijuana trafficking is generally punished less severely than cocaine. And state governments can set up their own criminal penalties and schedules for drugs as well.
Why does a drug's schedule matter?
A drug's schedule sets the groundwork for the federal regulation of a controlled substance.
Schedule 1 and 2 drugs face the strictest regulations. Schedule 1 drugs are effectively illegal for anything outside of research, and schedule 2 drugs can be used for limited medical purposes with the DEA's approval — for example, through a license for prescriptions.
The DEA even sets strict limits on the production of schedule 1 and 2 drugs, although the limits vary from drug to drug. Only one place in the US — a University of Mississippi farm — is currently allowed to grow marijuana under federal regulations, and the pot is limited to research purposes. By comparison, several private companies produce oxycodone, a schedule 2 substance, and use the drug for prescription painkillers.
A drug's schedule can interfere with state laws
A drug's schedule can interfere with state laws. Marijuana's schedule 1 status is one reason banks are reluctant to open accounts for pot shops and growers in Colorado and Washington, even though the businesses are legal under state law.
Federal tax law also prohibits businesses from deducting many expenses related to the trafficking of schedule 1 and 2 drugs, which can cause state-legal marijuana businesses' effective income tax rates to soar as high as 90 percent.
The DEA sometimes uses marijuana's classification to pressure physicians, hospitals, and pharmacies into not working with medical marijuana operations that are compliant with state law. If these medical providers don't comply, the DEA threatens to take back licensing that lets doctors prescribe drugs, such as prescription painkillers with oxycodone, that contain scheduled substances.
What does it take to reschedule a drug?
Congress could pass a law that changes or restricts a drug's schedule. But Congress mostly leaves scheduling to federal agencies like the DEA. (One exception: Congress previously passed the Hillory J. Farias and Samantha Reid Date-Rape Prevention Act of 2000 and added gamma hydroxybutyric acid, a date rape drug, to the scheduling system.)
The US attorney general can also initiate a review process that would look at the available evidence and potentially change a drug's schedule. The review includes several steps:
- The DEA, US Department of Health and Human Services, or public petition initiate a review.
- The DEA requests HHS to review the medical and scientific evidence regarding a drug's schedule.
- HHS, through the FDA, evaluates the drug and its schedule through an analysis based on eight factors. Among the factors: a drug's potential for abuse, the scientific evidence for a drug's pharmacological effects, and the scientific evidence for a drug's medical use.
- HHS recommends a schedule based on the scientific evidence.
- The DEA conducts its own review, with the HHS's determination in mind, and sets the final schedule.
Although very rigorous, this process has been successfully carried out in the past. For example, the DEA in 2014 announced it had rescheduled hydrocodone combination products, or opioid-based prescription painkillers, from schedule 3 to schedule 2.
'Almost 7 million Americans abuse controlled-substance prescription medications, including opioid painkillers, resulting in more deaths from prescription drug overdoses than auto accidents,' former DEA head Michele Leonhart said in a 2014 statement. 'Today's action recognizes that these products are some of the most addictive and potentially dangerous prescription medications available.'
Can a drug be unscheduled?
It's possible, but it's much more difficult than simply rescheduling a drug.
One big hurdle is international treaties. The US is party to international agreements that effectively require some drugs, including marijuana, to remain within the scheduling system — and possibly schedule 1 or 2.
Proving that a drug has no potential for abuse is also very difficult, if not impossible. An American Scientist analysis, for instance, found even relatively safe marijuana has some potential for dependence; it's less addictive than heroin, meth, cocaine, nicotine, and alcohol, but more addictive than hallucinogens such as LSD, which doesn't cause much, if any, dependence. And since pot is widely used recreationally, that makes it a sure lock-in for 'high potential for abuse.'
The two major recreational drugs not on the scheduling system — alcohol and tobacco — required a specific exemption in the Controlled Substances Act. Mark Kleiman, a drug policy expert, argues both would be marked schedule 1 if they were evaluated today, since they're widely used recreationally, addictive, detrimental to one's health and society, and deadly.
Why is marijuana still schedule 1?
When marijuana's classification comes under review, its schedule 1 status is consistently maintained due to insufficient scientific evidence of its medical value.
Specifically, the scientific evidence available for marijuana doesn't pass the threshold required by federal agencies to acknowledge a drug's potential as medicine. No studies proved the drug's medical efficacy in controlled, large-scale clinical environments. No studies established adequate safety protocols for marijuana. And marijuana's full chemical structure has never been characterized and analyzed.
There have been some studies showing marijuana has medical benefits, particularly for pain and muscle stiffness. But these studies haven’t been large enough to meet the threshold the DEA and other federal agencies, such as the FDA, require to prove a drug has medical value — by proving its worth in controlled, large-scale clinical trials.
But one reason there isn't enough scientific evidence to change marijuana's schedule 1 status might be, in fact, the drug's schedule 1 status. The DEA restricts how much marijuana can go to research. To obtain legal marijuana supplies for studies, researchers must get their studies approved by HHS, the FDA, and the DEA.
Changing marijuana's schedule, in other words, is a bit of a Catch-22. There needs to be a certain level of scientific research that proves marijuana has medical value, but the federal government's restrictions make it difficult to conduct that research.
To address those issues, the DEA hopes to allow much more research into pot in other ways. For one, it’s increased the amount of pot grown for research over the past few years, and it plans to continue doing so. Crucially, it also plans to let more people and facilities grow marijuana for studies — aside from University of Mississippi, the only federally legal grower right now.
That could significantly open up research access to pot — including potentially higher-quality marijuana and different strains of the drug, which the University of Mississippi doesn’t currently meet demands for. But the effects of the changes remain to be seen.
While a reclassification would be a symbolic win for legalization advocates, Kleiman says it wouldn't have much practical effect. Schedule 2 substances typically require a prescription to be distributed, and the state-legal marijuana dispensaries and retail outlets don’t work through traditional prescriptions (they distribute 'recommendations' for medical marijuana) — so even rescheduling may not open up access. (Cocaine and meth are schedule 2, and they’re definitely not easily legally available, after all.)
Still, if the federal government acknowledged pot’s medical value through a schedule 2 classification, advocates hoped it would make federal agencies far more receptive to paying for and approving medical research into pot. But the DEA hopes its other steps will unlock far more research instead.
There would be some effects on policy, such as allowing state-legal marijuana business to deduct certain taxes, if marijuana was reclassified to schedule 3 or lower. But that’s extremely unlikely: Schedule 3 and lower drugs need to have some medical value and not meet criteria for 'high potential for abuse.' Since marijuana is widely used recreationally, it’s a lock-in for 'high potential for abuse,' keeping it at schedule 1 or 2.
Is there an alternative to the scheduling system?
Kleiman has proposed moving to a scheduling system that looks only at a drug's potential for abuse without considering whether it has medical value. The regime would control all intoxicating drugs, including alcohol, to try to prevent problematic drug use, based on the scientific definition of drug abuse disorders. Whether a drug has medical value would be addressed by a different set of policies focused on medical drug production and health care.
'Whether something is medication is a separate set of issues,' Kleiman said. His ideal system 'would classify drugs by dangerousness, with a penalties for dealing ramping up with more dangerous drugs.'
One of the problems with the current scheduling system, Kleiman argued, is it tries to lump drugs with completely different effects and risks into a few categories. 'There are lots of different drugs in the world, and we have to figure out what to do with each of them,' Kleiman said. 'Some categorization is helpful, but it's not as if the drugs neatly break down.'
Kleiman, who supports decriminalizing illicit drug use and legalizing marijuana, said an ideal replacement to the current scheduling system would also come with less stringent criminal penalties.
'The current prohibition system has generated many bad side effects,' Kleiman said. 'We should try to develop new policies that duplicate the success of preventing the development of more drugs that are as big a problem as alcohol, but with fewer of the costs.'